Remdesivir cleared for use in treating most severe COVID-19 cases in India!
The COVID-19 Novel Corona Virus Pandemic is still going strong in most countries and many teams of researchers and scientists are working round-the-clock to identify a cure or vaccine for this. However, there have been a significant number of people who have recovered from the virus after being treated with various drugs. Earlier, Hydroxychloroquine was considered to be very effective against this virus but it had a number of side effects. Later, the anti-viral drug was looked upon as the answer but it was still not approved for widespread usage. However, India's top drug regulatory body - Drug Controller of India has now approved the usage of Remdesivir to treat severe cases of the COVID-19!

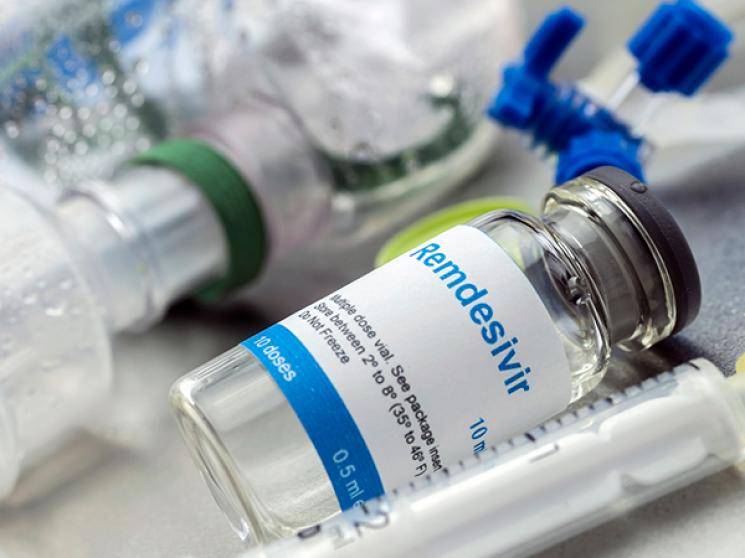
However, Remdesivir has only been approved for a maximum usage of a 5-day regimen, according to reports. The Central Drugs Standard Control Organization has decided against extending the use of Remdesivir to 10 days, after examining the reports presented to it, at the time of approving its usage for 5 days. This drug is currently being manufactured in the USA by Gilead Sciences and would be imported to India by the Mumbai-based Klineria Global Sciences. With the approval from the authorities, now generic voluntary licence holders in our country can go ahead with manufacturing the drug. Keep watching this space for further updates...
தனுஷ் பாடலுக்கு டிக்டாக் செய்த டேவிட் வார்னர் !
02/06/2020 10:52 AM
பன்மொழிகளில் உருவாகும் கர்ணம் மல்லேஸ்வரி வாழ்க்கை வரலாற்று திரைப்படம் !
02/06/2020 09:56 AM
ரீமேக் ஆகிறதா அவள் அப்படிதான்...விவரம் இதோ !
01/06/2020 07:43 PM
சிவப்பு மஞ்சள் பச்சை படத்தின் OST Volume 1 வெளியீடு !
01/06/2020 06:40 PM
Load More
About This Page
People looking for online information on will find this news story useful.