
The leading health body in the country for the coronavirus treatments, the Indian Council for Medical Research (ICMR), has asked the state governments to return the rapid antibody testing kits due to non-satisfactory results. The Centre had acquired over 5 lakh testing kits from two Chinese companies - Guangzhou Wondfo Biotech and Zhuhai Livzon Diagnostics - with the ICMR ordering for testing to be halted last week owing to inconsistencies in the results that were being carried out by medical and health officials across various states in the country.
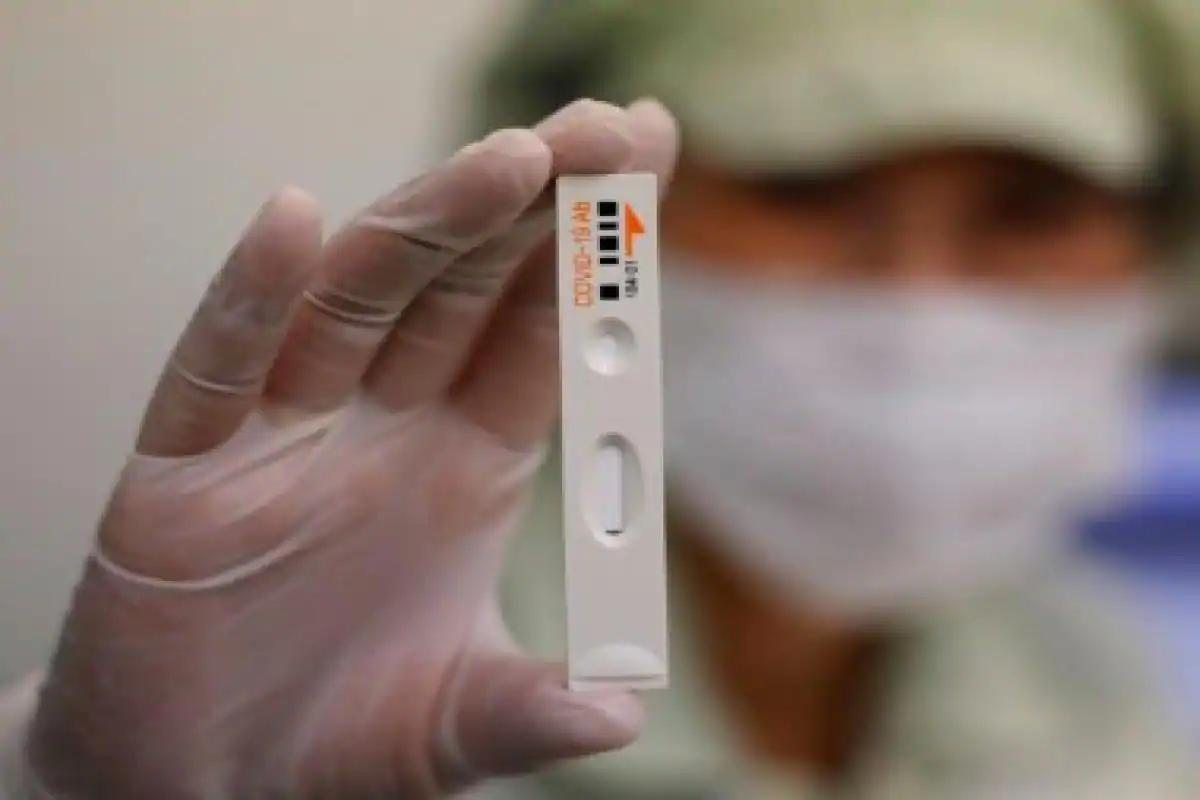
The ICMR had stated that variations were noticed in the results that were being shown in the rapid testing kits acquired from the manufacturers, Guangzhou Wondfo Biotech and Zhuhai Livzon Diagnostics. Furthermore, the ICMR in a letter to the chief secretaries of all states and Union territories has informed that it approves of the swab RT-PCR (Reverse transcription polymerase chain reaction) test to identify the presence of the coronavirus in the human body and is "the best use for diagnosis" at an early stage.

வாத்தி கம்மிங் ஒத்து நடனத்தில் வெளுத்து வாங்கும் ஷில்பா ஷெட்டி !
26/04/2020 06:07 PM
இன்ஸ்டாகிராமில் இணைந்த மாஸ்டர் இயக்குனர் !
26/04/2020 06:03 PM
அறிவும் அன்பும் உருவான கதை குறித்து ஜிப்ரான் வெளிப்படை !
26/04/2020 06:00 PM
மாஸ்டர் ட்ரைலர் குறித்து லோகேஷ் கனகராஜ் பதிவு !
26/04/2020 05:57 PM
பிறந்தநாள் கொண்டாட்டம் குறித்து ரசிகர்களுக்கு அன்புகட்டளையிட்ட தல அஜித் !
26/04/2020 01:58 PM
நேரடியாக OTT-யில் வெளியாகிறதா மாஸ்டர்...? விஜய் தரப்பு விளக்கம் இதோ
26/04/2020 10:58 AM
























